

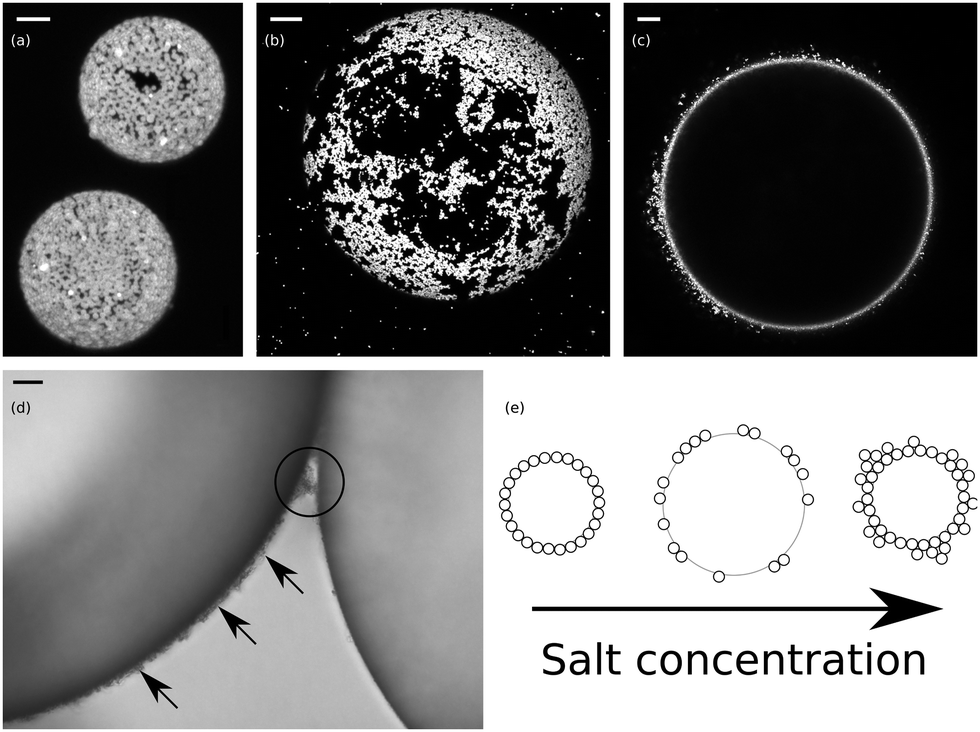
We show that the butyl analogue behaves similarly to that of cetyltrimethylammonium bromide surfactant with respect to promoting silica particles to emulsion drop interfaces. Nanogels made from crosslinked block copolymer micelles are used as stabilizers in the Pickering emulsion polymerization of styrene. The three-phase oil-water-solid contact angle increases with salt concentration and R chain length, confirming the increase in particle hydrophobicity on addition of salt. This system contains -Cyclodextrin (-CD) and a natural origin selected surfactant, Polyglyceryl-3 diisostearate (PG3D). No stable emulsion forms for the methyl analogue, but very stable o/w emulsions can be prepared with the other three members, with the minimum concentration of salt being required decreasing with R chain length to as low as 5 × 10 -5 M. A new oil-in-water (O/W) emulsifying system suitable for use in preparing Pickering emulsions with a large range of oils was developed. Unlike inorganic electrolytes, the addition of these salts does not lead to particle flocculation in water, although the particle charge is reduced. Substituting solid particles for traditional surfactants, Pickering emulsions are more stable against coalescence and can obtain many useful properties. The chain length of the R group is systematically increased from 1 (methyl) to 4 (butyl). Pickering emulsion, a kind of emulsion stabilized only by solid particles locating at oilwater interface, has been discovered a century ago, while being extensively studied in recent decades. Here, we describe the effects of adding tetraalkylammonium salts (R 4NX, X is an anion) to aqueous dispersions of silica nanoparticles at high pH and investigating the possibility of subsequently stabilizing octane-in-water (o/w) emulsions. (B) Confocal micrograph of an o/w emulsion stabilised by wax particles red colour represents fluorescence signal from the dyed colloidal wax particles Zafeiri, I., et al. In most cases, inorganic salts are added, while little attention has been paid to the use of organic salts. One way to promote particle adsorption to the oil-water interface is to add salt to the aqueous phase, although particle flocculation normally ensues. a 1 a 4 Bright-field and confocal fluorescence microscopy images of the emulsion droplets with inner oil and water phases dyed by Nile Red and FITC-Dextran respectively observed at 530 nm (a 2. At high pH, bare silica particles are not an effective Pickering emulsion stabilizer of nonpolar oils with water due to their high surface charge.


 0 kommentar(er)
0 kommentar(er)
